Wild bees in the agricultural landscape
Causes of endangerment, habitat requirements and approaches for the integration of biotope structures into an agroforestry systemThe article was written as a term paper as part of Anna-Lea Ortman's Master's degree in Landscape Ecology, who kindly made the work available to us for our blog.
1. introduction
1.1 Wild bees in Germany
In addition to the domesticated European honey bee (Apis mellifera), which is mainly kept by beekeepers for honey production, there are more than 566 species of wild bees in Germany. These can be divided into 41 genera, of which masked and silk bees, sand and furrow bees, wool and resin bees, mason and leafcutter bees and bumblebees, the best-known wild bee genus, represent only a fraction (WESTRICH 2018:405).
Willow sand bee
Wild bees are also increasingly under threat and many species are declining in their populations, joining the ranks of insect extinction now perceived by the public and the media.
Over half of wild bee species are on the Red List for Germany (BfN 2017).
In 2011, 7 % of species were considered extinct (0), 40.9 % as endangered (1, 2, 3, G) and 4.7 % as extremely rare (R). A further 7.5 % are on the early warning list (V) and only 37.2 % of wild bee species are classified as endangered (*) according to the current Red List status. Data is insufficient for the remaining 2.7 % (WESTRICH et al. 2011).

Mason bee in front of nesting site
1.2 Causes of endangerment & potential in the agricultural landscape
Over the last two centuries,specialization, segregation and homogenization of agricultural production have led to a largely intensively used agricultural landscape. This development has resulted in a sharp decline in the structural and biological diversity of the agricultural landscape (GARIBALDI et al. 2013, HABER 2014:86). The current dominant agricultural practices hardly meet the habitat requirements of wild bee species, which are highly specialized in many respects. There is currently a serious lack of food sources and nesting sites (WESTRICH 2018:7). At the same time, agriculture, which also has a landscape conservation component, offers many starting points for contributing to insect conservation in general, and wild bee conservation in particular. In view of the sharp decline in insect diversity and total biomass in the landscape(HALLMANN et al. 2017), biodiversity conservation should be taken into account alongside agronomic aspects when designing crop rotation, selecting crops and crop management. By reducing or eliminating the use of plant protection products - especially insecticides and herbicides - negative effects on wild bees can be directly and indirectly reduced or avoided(FREEMARK & BOUTIN 1995, RUNDLÖF et al. 2015, WESTRICH 2018:46, HABEL et al. 2019). By preserving landscape elements, creating flower strips and other biotope structures, farmers can make an additional contribution to the conservation value of agricultural landscapes for wild bees (RUNDLÖF et al. 2015). Measures that are spatially limited to nature reserves or national parks will not be sufficient for successful wild bee conservation.According to the metapopulation theory published by HANSKI in 1999, the conservation of specialized species in particular depends on the exchange of individuals and genes between different subpopulations across protected areas (WESTRICH 2018:86). While the predominantly poor habitat quality in large parts of the landscape contributes to habitat fragmentation (RUNDLÖF et al. 2007, HABEL et al. 2014), biotope connectivity elements of sufficient quantity and quality can promote the networking of sub-habitats and the exchange between sub-populations (WESTRICH 2018:13). Wild bee conservation therefore requires a level of consideration and impact that includes the entire landscape context. Integrative nature conservation that understands and implements biotope protection as a permanent part of area-wide agricultural practice therefore appears to be of paramount importance (GARIBALDI et al. 2013, WESTRICH 2018:9).
1.3 Protecting wild bees - why?
On the one hand, the case for protecting wild bees can be made from a biocentric perspective. According to this perspective, every species has an intrinsic value that requires its survival to be made possible, regardless of human benefit. In addition, an anthropocentric perspective emphasizes the importance of protecting insects and wild bees. Pollinating wild insects - including wild bees - make an enormous contribution to securing the world's food supply, maintaining biodiversity and ecosystem stability(POTTS et al. 2016). According to estimates by AIZEN et al. (2009), the loss of pollination services by insects and other pollinators, including vertebrates (e.g. birds and bats) in tropical countries, would result in global yield losses of 5 - 8 %. LAUTENBACH et al. (2012) calculated from 2009 figures that pollination by animals increases global annual harvests by 235 - 577 billion US$ in monetary terms. Bees are considered the most important group of pollinators, as they are able to pollinate 90 % of the 107 most cultivated agricultural crops (POTTS et al. 2016). The exact share of wild bees and other wild insects in global pollination services often appears to be underestimated. GARIBALDI et al.(2013) found in studies distributed across the globe that wild insects pollinate more effectively than honey bees (Apis), which are domesticated and specifically used by humans. A wild insect individual visiting a flower was twice as likely to successfully pollinate the crop compared to a honey bee individual. Wild insects increased the fruit set and thus potentially the crop yield regardless of the abundance of honey bees (GARIBALDI et al. 2013).
According to this, the targeted use of honey bees can additionally promote pollination performance, but cannot substitute the pollination services of the diversity of wild insects with the same efficiency. Honey bees are not able to adequately pollinate every crop species and are considered unreliable pollinators in cold and wet weather(GOULSON 2003). GARIBALDI et al. (2013) therefore recommend the targeted promotion of wild bees and the utilization of the complementarity of different pollinator insects. In addition, a diversity of pollinating insects is a kind of insurance for the agroecosystem, as species that are lost in space and time can be replaced by functionally redundant species. This serves the overarching goal of ensuring long-term yield security and yield stability(YACHI & LOREAU 1999, LIERE et al. 2017).
1.4 Competition between wild bees and honey bees?
The extent to which there is competition for food resources between wild bees and honey bees is the subject of current research. While the honey bee was not identified by STEFFAN-DEWENTER & TSCHARNTKE (2000) as a significant competitor of the wild bee fauna studied, other studies have shown negative influences of honey bee husbandry on the diversity(HUDEWENZ & KLEIN 2013), reproduction and growth(WOJCIK et al. 2018) of wild bees. The competitive relationships appear to be influenced by the availability of food resources. This highlights the relevance of a sufficient pollen supply in the agricultural landscape (WOJCIK et al. 2018).
Bumblebeeson blackberry blossoms
2. habitat requirements, lifestyle and reproduction of wild bees
In addition to climatic requirements, various habitat requirements must be met within a landscape for it to be successfully colonized by wild bees.As shown schematically in Figure 1, firstly, there must be sufficient foraging habitat with pollen and nectar plants. The majority of wild bee species are polylectic in their pollen collecting behavior (pollen generalists). However, 30 % of wild bee species are dependent on the pollen of a specific plant family, genus or even species. They are referred to as oligolectic (pollen specialists).
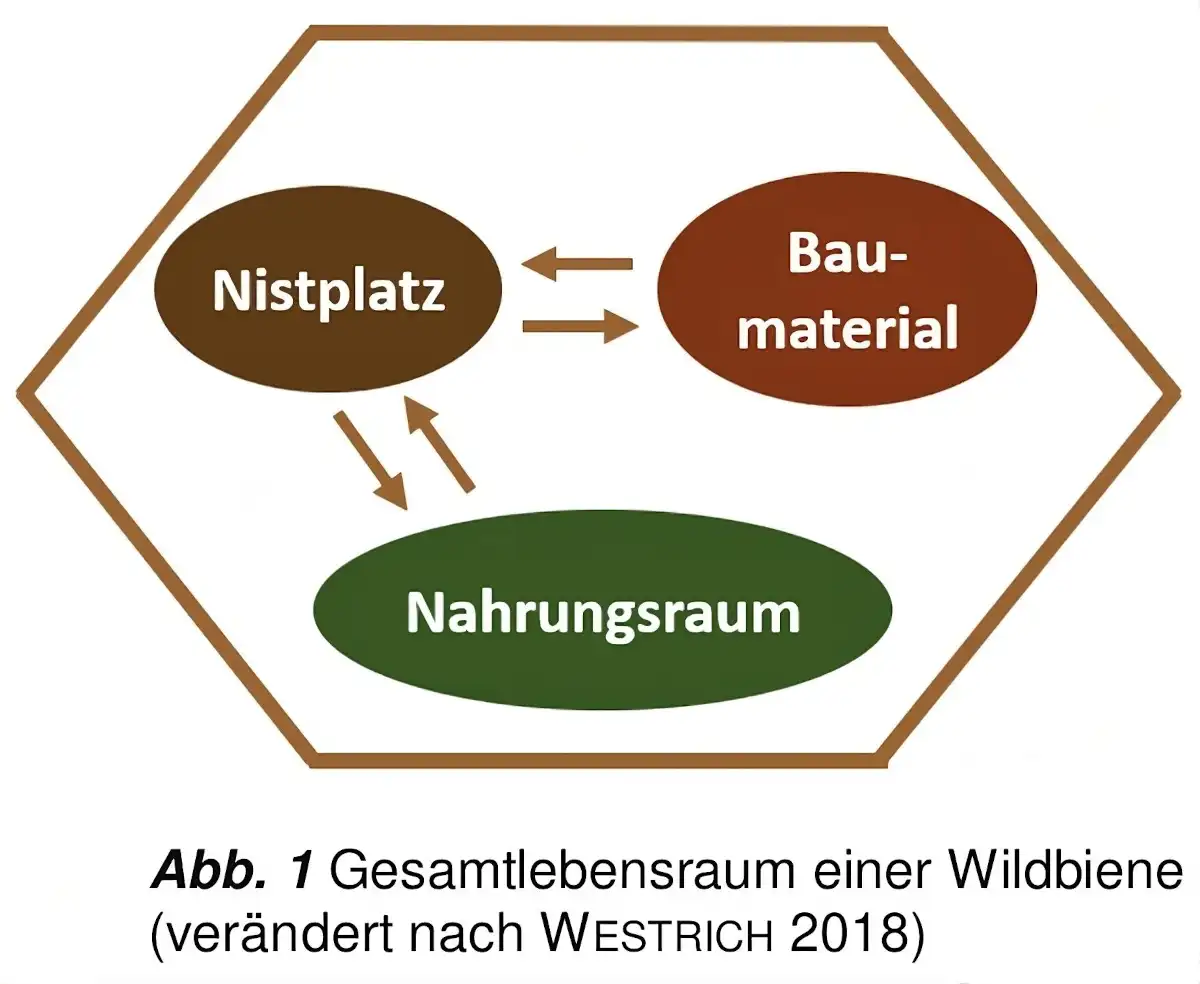
Wild bees are usually less choosy when it comes to nectar sources, which are mainly used for their own energy supply(DWS 2021). A sufficient and varied supply of nectar and pollen should be available throughout the entire growing season(WESTRICH 2018:25). This is a prerequisite for fitness and successful reproduction. Suitable structures as nesting sites and suitable building materials for their construction are a prerequisite for the latter.
If the various habitat factors are not fulfilled in one place, different partial habitats can be used if they are sufficiently connected and accessible within the species-specific radius of action (WESTRICH 2018:13). The exact habitat requirements vary from species to species. Section 3 presents an outline of various habitat structures and their suitability for certain wild bee species. As the ecology of the individual wild bee species is very individual, only part of the habitat requirements of each species can be described here.
Most wild bees live solitary (solitary bees). Only bumblebees (Bombus) and some species of furrow bees (Halictus) have a social lifestyle. Wild bees are only 4 - 6 weeks old, so they only have a short period of time to reproduce successfully. In solitary bees, the males are the first to hatch. After mating, the solitary female wild bee begins to build a brood chamber for her own brood.
A female wild bee builds 4 - 30 brood cells in her few weeks of life. One by one, she lays an egg, as well as nectar and pollen as food rations for the hatching larvae. The individual brood cells are sealed with chewed plant parts, clay, sand, small stones or tree resin and thus protected as far as possible from the weather and predators. After about a year, the larvae have undergone holometabolous development into imago and crawl out of the nest for the first time.
Around a quarter of all wild bee species in Germany are so-called cuckoo bees.
These do not carry out their own brood care. A female cuckoo bee lays her eggs in the finished nest of another female bee. The cuckoo larvae hatch before the offspring of the host bee and feed on the pollen supplies actually intended for the latter. The host larvae that hatch later starve to death.(DWS 2021)
3. potential habitat structures of the agricultural landscape and proposals for the implementation of measures in an agroforestry system
Map 1 below shows a one-hectare agroforestry system that illustrates the possibilities for integrating nature conservation objectives into land use.The agroforestry system combines the polycultural cultivation of nut and fruit crops with vegetables and cut flowers according to the market gardening principle in permanent beds.
In addition, biotope structures for various species groups are to be created and maintained in parts of the area. Proposals for measures to enhance nature conservation and suitable areas in the context of land use were presented with various hatchings (
measures are intended to promote wild bee diversity and abundance, but in many respects would also enhance the value of other species groups.
3.1 Fields
Wild bee species found in the agricultural landscape mainly use fields to collect pollen and nectar from flowers of the segetal flora and some cultivated plants. Only a few species create nesting sites within the cultivated areas. Tillage acts as a limiting factor here. Most wild bee species therefore primarily use field edge structures such as hedges, copses, edges or adjacent meadows as nesting sites, where there is hardly any tillage. Such edge structures are found in higher densities in small-scale agricultural landscapes than in those with large fields.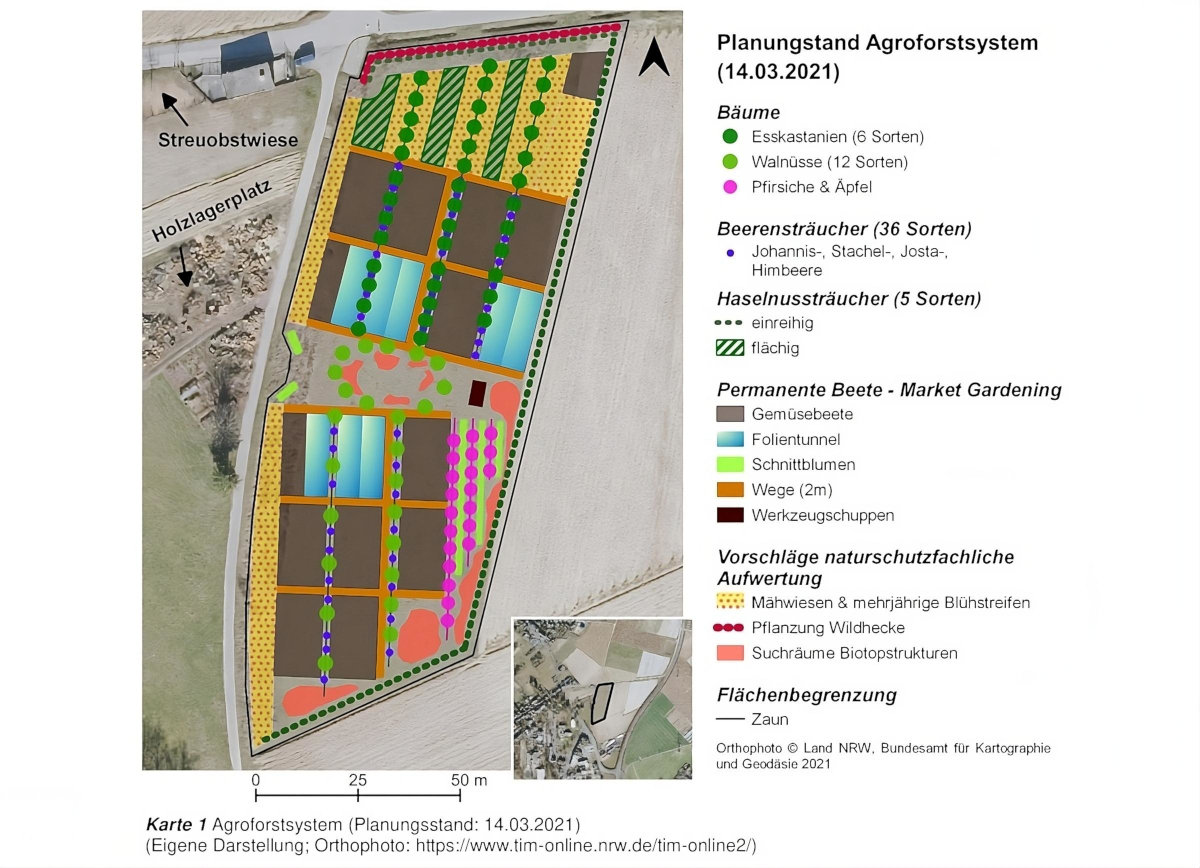
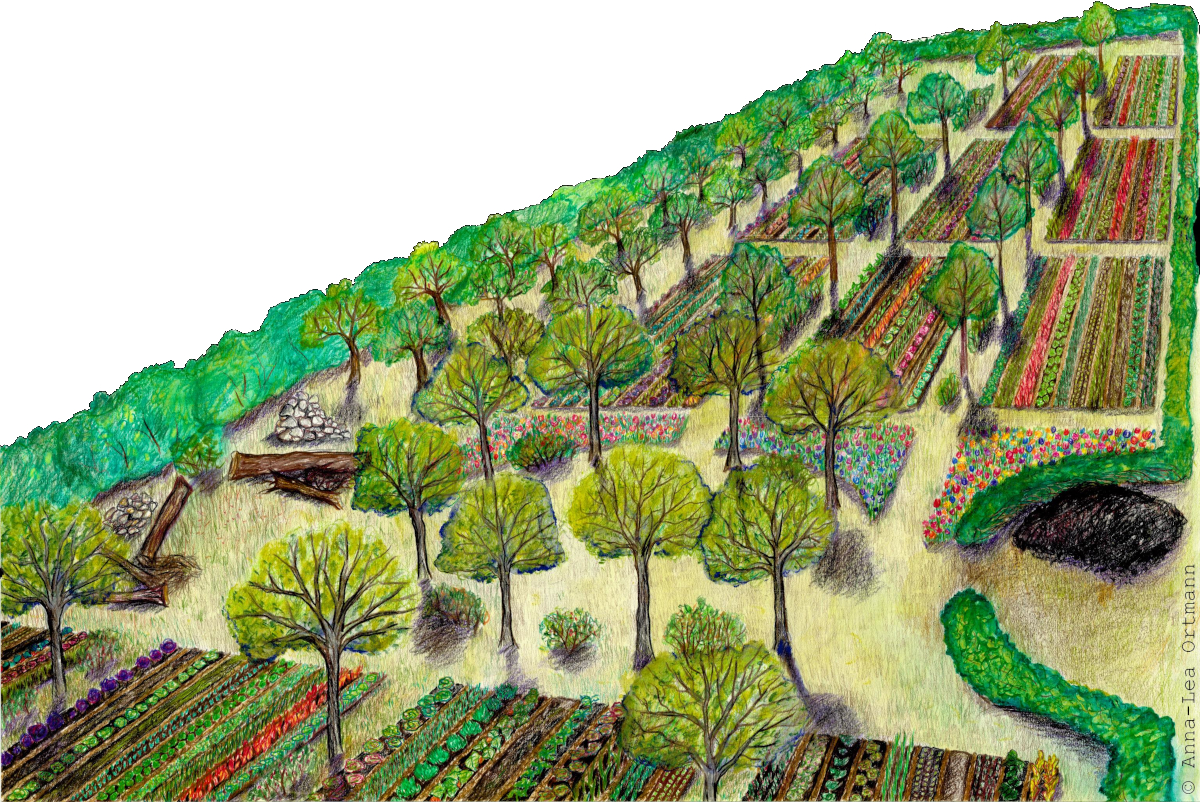
Illustration of an agroforestry system
3.2 Hedges
Hedges provide valuable structures for numerous wild bee species. On the one hand, their flowers provide food, and on the other, dry stems and beetle feeding galleries in branches and wood provide nesting opportunities for some wild bee species. Species that characteristically benefit from hedges are the masked bee species Hylaeus brevicornus and H. Communis and the sand bee species Andrena bicolor and A. Chrysoceles. The mason bee Osmia leucomelana and various bumblebee species (Bombus) also like to build their nests in the protection of the hedge (WESTRICH 2018:25). The creation of a hedge of native shrub species was considered for the northern part of the area when planning the agroforestry system.

Native species typical of the natural habitat, such as Prunus spinosa, Cratageus laevigate and C.
Monogyna, as well as Salix spec. Salix caprea in particular provides a high quality habitat for many wild bee species (WESTRICH 2018:389). The planting material should be obtained from autochthonous populations wherever possible. Hedgerows and hedgerows have disappeared in many places, although they provide wind protection for cultivated areas and contribute to the preservation of agricultural biodiversity. Staggering the hedgerow at different heights and creating fringe-like transitions create additional microhabitats and niches. Due to their historically diverse design and maintenance, hedges are considered to be valuable elements of cultural and landscape history today (REIF et al. 2004).


3.3 Plants as a source of pollen, nectar and building material
Since about one third of the wild bee species found in Germany specialize in the Bee Pollen (Bio) of a particular plant family, genus or even species, the promotion and preservation of the entire diversity of wild bees is linked to the greatest possible variety of available food plant species. To illustrate the specialized attachment of wild bee species to certain plant species, a few examples are given below. The earth or sand bee Andrena florea specializes in the red-berried (Bryonia diocia) and white bryony (Bryonia alba) as pollen sources and is only found where these plants flower regularly (WESTRICH 2018:462). The meadow knautia (Knautia arvensis) is considered a particularly important pollen source for Andrena hattorfiana (WESTRICH 2018:28).The common viper's bugloss (Echium vulgare) is the only pollen source in Germany for the mason bee species Osmia anthocopodis (WESTRICH 2018:14). Red clover (Trifolium pratense) and other forage legumes from the Fabacea family provide attractive nectar and pollen sources for long-trunked bumblebee species (WESTRICH 2018:46). Lamiaceae can also often be better utilized by long-trunked bumblebees than by many other groups of wild bees. Centaurea cyanus is an attractive pollen source for the polylectic mason bee Osmia papaveris, along with some other segetal and ruderal species (WESTRICH 2018:45). In turn, this species prefers to use pieces of poppy petals (Papaver rhoes) to line its brood cells (WESTRICH 2018:47).
The wild herbs mentioned above could be promoted on the site through extensive mowing management (section 3.4.) or autochthonous seeding (3.5.). The fence around the agroforestry system, which is several hundred meters long, could be planted with climbing red-berried or white fenugreek or other species that serve as food plants for wild bees (e.g. Calystegia sepium, Phaseolus coccineus).

Common viper's bugloss
3.4 Mowed meadows
Floristically species-rich, extensive mown meadows are valuable food resources for wild bees. At the same time, where loose, semi-open patches of ground are present, they offer potential areas for the creation of breeding chambers. Extensive mowing management can increase the nature conservation value of grassland. In order to promote and maintain a colorful meadow flora, WESTRICH (2018:32) recommends regular two-furrow mowing with a hand mower, brush cutter or bar mower. The first cut should be carried out between mid-June and early July, the second cut at the end of August or September. Where biodiversity is to be promoted, frequent cutting (e.g. by lawnmowers) should be avoided, as many meadow herbs cannot tolerate this (WESTRICH 2018:32). The biodiversity of meadows tends to be higher with lower nutrient levels, so nutrient removal (removal andmulching/composting elsewhere) is preferable to nutrient enrichment (mulching on site). Leaching can only be achieved through long-term maintenance. Completely avoiding mowing would not be conducive to the promotion of wild bees, as plants with weak competition would disappear from the meadow and the biotope would become overgrown in the long term. Staggered mowing in strips or mosaics ensures that flowering and refuge areas (10 - 20 % of the area or 1 - 6 m wide strips) always remain on the site and are not completely removed with one mowing date. Removing the mowed material from the meadow areas is also important to prevent new plant growth from being hindered and existing bee nests in the soil from being covered for returning female bees (WESTRICH 2018:29).
3.5 Flower strips
Where arable use takes place, the creation of flower strips in a narrow section of the field offers the opportunity to enhance the landscape for wild bees.
Wildflower meadow at the Aspermühle
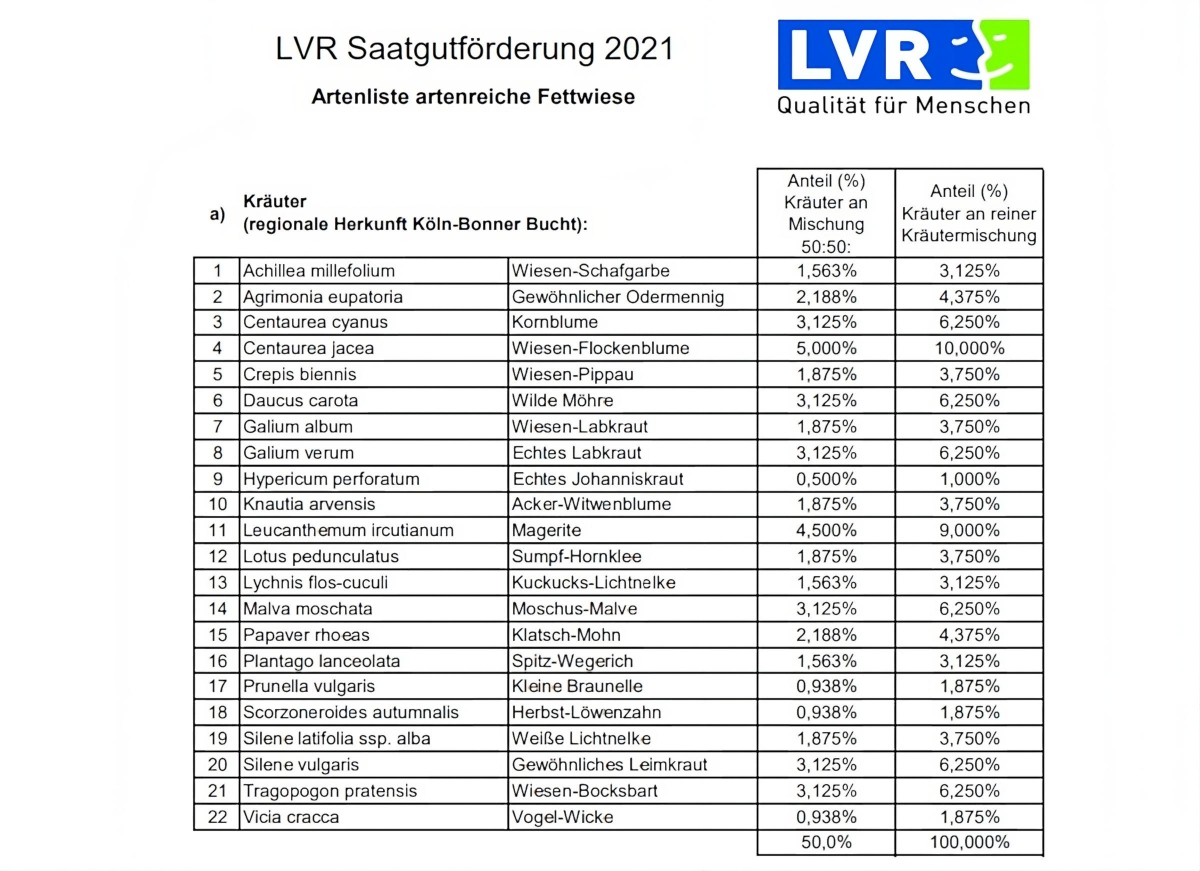
As wild bees are site-loyal, it is particularly important that they remain in place for several years(SHARAF 2018). The choice of autochthonous seeds with species typical of the region is considered sensible from a nature conservation perspective and prevents flora distortion (CHMELA 2021). In addition, the flowering mixture should offer the greatest possible variety of forage plants suitable for specialists (WESTRICH 2018:46). Many of the herb species mentioned in section 3.3. that are valuable for wild bee species can also be found in the seed mixtures of the Landschaftsverband Rheinland (LVR ) that are typical for the Cologne-Bonn Bay region (see list in the appendix). In addition to the supply of pollen and nectar, flower strips also have a beneficial effect on ground-nesting species due to their perennial dormancy.
Breeding and overwintering are more likely to be successful here than in ploughed, cultivated, hoed or harrowed arable areas. Flower strips or
areas with an extensive mowing regime would be suitable on the site, particularly in the north between the planted hazelnut strips and along the western boundary of the fence. Autochthonous seeds could be obtained from the RegioSaatGut project of Biostation Bonn/ Rhein-Erft e.V., which cooperates with the LVR (CHMELA 2021).
3.6 Fruit and nut trees
Various mason bee species use the pollen from fruit trees and also act as good fruit pollinators. The species Osmia cornuta and O. Rufa are sometimes specifically bred for this purpose. Osmia bicornis collects pollen from apple blossoms in particular, but also uses Bee Pollen (Bio) from the walnut Juglans regia.

One hectare of apple orchard provides pollen for the rearing of around 22,000 individuals (WESTRICH 2018:25). Various bumblebee species also use fruit blossoms as pollen sources. Bumblebees are less flower tolerant than honeybees. This is an advantage for apples and sweet cherries, as the bumblebee is generally more effective as a pollinator in these fruit crops, which are dependent on cross-pollination, due to more frequent switching between different varieties. As the age of the fruit and nut trees in the agroforestry system increases, the amount of pollen available will increase. It is hoped that wild bee species foraging on fruit blossoms will migrate from the adjacent orchard meadow to the northwest, which is covered with older trees, and colonize the agroforestry system (Map 1).

Quince blossoms provide high-quality pollen and nectar
3.7 Areas with little vegetation and other small structures
Small structures are of great importance for many wild bee species. Numerous ground-dwelling species depend on horizontal to gently sloping open ground or areas with sparse vegetation as a place to nest (WESTRICH 2018:63). WESTRICH (2018:9) emphasizes that natural processes and disturbances such as erosion, earth movements, open horizontal and vertical soil structures and flat pioneer stages should be tolerated more in the agricultural landscape wherever possible in order to protect bees. Depending on the species, loose or firmer sand, gravel or clay areas are used. Favorable small structures can be found, for example, along roadsides with sparse vegetation or on embankments. Expertly constructed dry stone walls and cairns can provide nesting sites for Osmia anthocopodis or O. Ravouxi, for example (WESTRICH 2018:14,66). The vertical outcrops of earth between the stones of walls can create structures similar to rock faces, so that they function as secondary habitats. At the same time, rarer plants can survive on such walls, whose flowers provide additional food. Deadwood and decaying wood - especially from deciduous trees - can provide further biotope structures and nesting opportunities. The wood bee species Xylocopa violaceae, for example, uses tree roots and wooden beams to create brood chambers. Depending on their diameter, beetle feeding galleries in dead wood are used by a wide variety of masked bees (Hylaeus). Abandoned small mammal burrows, such as mouse nests and mole burrows, are used by the dark and light ground bumblebee Bombus terrestris and B. Lucorum to build bumblebee nests in them. Among the mason bees (Osmia), some species are obliged to build their nests in empty snail shells. For example, the snail-shelled mason bee Andrena bicolor breeds in the shells of various species, e.g. from the genus Cepaea.
Wood bee
Andrena bicolor as well as the other mason bee species that rely on snail shells have their settlement focus outside of intensively used parts of the agricultural landscape on nutrient-poor grasslands, weathered slopes, on rocky slopes and on south-facing forest edges. (WESTRICH 2018: 58, 67, 68, 710)
The creation of small structures could take place within the search areas for biotope structures shown in Map 1. The preservation of open ground areas requires the removal of vegetation if it covers more than half of the area to be kept clear. Maintenance interventions should be limited to what is necessary and take place in September and October (WESTRICH 2018: 59). Deadwood and stone elements could also be integrated into these search areas. Due to the existing structures on the residual wood storage area to the west of the agroforestry system, it is to be hoped that species dependent on deadwood in particular are already present in close proximity.
4. outlook: Potentials and further action for nature conservation enhancement
The agroforestry system already has exciting structures in terms of nature conservation.Sub-areas that are not farmed offer potential for the creation of biotope structures. Long-term maintenance of these is of great importance, as some biotopes (e.g. permanent flowering strips) only become fully established after several years of maintenance and their nature conservation value increases over time.
Before implementing initial nature conservation measures, a further step should be to examine the area and carry out research across the group of wild bees in order to answer the following questions:
- Which species (potentially) occur on the area and should be promoted?
- Where are there internal conflicts between the Where are there internal conflicts between the protection of different species (groups) with regard to the design of biotope structures?
- Where can synergies be identified with the horticultural use of the area (e.g.
promotion of beneficial insects)?
- Where can synergies with the horticultural use of the area be identified (e.g. promotion of beneficial organisms)?
- What are the conflicts with land use?
In order to answer these questions in depth, animal and vegetation ecology surveys would be necessary. Monitoring the area and its structures would also be valuable in the long term, for example to track developments in wild bee diversity.
Appendix 5
6. literature
AIZEN, M. A., GARIBALDI, L. A., CUNNINGHAM, S. A., & A. M. KLEIN (2009): How much doesagriculture depend on pollinators? Lessons from long-term trends in crop production. Annals of botany, 103(9), 1579-1588.
BfN (2017): "BienABest". Wild bees in focus. Joint press release with the Federal Ministry for the Environment. Berlin/Bonn, May 29, 2017.
https://www.bfn.de/presse/pressearchiv/2017/detailseite.html?tx_ttnews%5Btt_news%5D=60 80& (last call: 12.03.2021) CHMELA, C. (2021): Oral communication on the RegioSaatGut project of Biostation Bonn / Rhein-Erft e.V.
DWS (2021): WILDBIES. Important pollinators for a variety of plants.
https://www.deutschewildtierstiftung.de/wildtiere/wildbienen (last call: 12.03.2021) FREEMARK, K. & C. BOUTIN (1995): Impacts of agricultural herbicide use on terrestrial wildlife in temperate landscapes: review with special reference to North America. Agric Ecosyst Environ, 52, 67-91.
G ARIBALDI, L. A., AIZEN, M. A., KLEIN, A. M., CUNNINGHAM, S. A. & L. D. HARDER (2011): Global
Growth and Stability of Agricultural Yield Decrease with Pollinator Dependence. Proceedings of the National Academy of Sciences, 108(14), 5909-5914.
G ARIBALDI, L. A., STEFFAN-DEWENTER, I., W INFREE, R. , AIZEN, M. A., BOMMARCO, R.,
CUNNINGHAM , S. A., KREMEN, C., CARVALHEIRO, L. G., HARDER, L. D., AFIK, O., BARTOMEUS, I.,
BENJAMIN, F., BOREUX, V., CARIVEAU, D., C HACOFF, N. P., DUDENHÖFFER, J. H., FREITAS, B. M.
, GHAZOUL, J., GREENLEAF, S., HIPÓLITO, J. , HOLZSCHUH, A., H OWLETT , B., ISAACS, R.,
JAVOREK, S. K., K ENNEDY, C. M., KREWENKA, K., KRISHNAN, S., MANDELIK, Y., MAYFIELD, M.
M., MOTZKE, I., M UNYULI, T., NAULT, B. A., OTIENO, M., PETERSEN, J., PISANTY, G., POTTS, R.
RADER, S. G., RICKETTS, T. H., RUNDLÖF, M., SEYMOUR, C. L., SCHÜEPP, C., SZENTGYÖRGYI,
H., TAKI, H., TSCHARNTKE, T., VERGARA, C. H., VIANA, B. F., W ANGER, T. C., W ESTPHAL, C.,
WILLIAMS N. & A. M. KLEIN (2013): Wild Pollinators Enhance Fruit Set of Crops Regardless of Honey Bee Abundance. Science, 339(6127), 1608-1611.
G OULSON, D. (2003): Conserving wild bees for crop pollination. Journal of Food Agriculture and Environment, 1, 142-144.
G OULSON, D., LYE, G. C., & B. DARVILL (2008): Decline and conservation of bumble bees. Annu. Rev. Entomol., 53, 191-208.
HALLMANN, C. A., SORG, M., JONGEJANS, E., S IEPEL, H., HOFLAND, N., SCHWAN, H., STENMANS,
W., MÜLLER, A., SUMSER, H., HÖRREN, T., GOULSON, D. & H. DE KROON (2017): More than 75
percent decline over 27 years in total flying insect biomass in protected areas. PloS one, 12(10), e0185809.
KLEIN, A.-M., VAISSIÈRE, B. E., CANE, J. H., STEFFAN-DEWENTER, I., CUNNINGHAM, S. A.,
KREMEN, C. & T. TSCHARNTKE (2007): Importance of Pollinators in Changing Landscapes for World Crops. Proceedings of the Royal Society B: Biological Sciences, 274, 303-313.
H ABEL , J. C., SAMWAYS , M. J., & T. S CHMITT (2019): Mitigating the precipitous decline of terrestrial
European insects: Requirements for a new strategy. Biodiversity and Conservation, 28(6), 1343-1360.
H ABER , W. (2014): Agriculture and nature conservation. John Wiley & Sons.
H ANSKI , I. (1999): Habitat connectivity, habitat continuity, and metapopulations in dynamic landscapes. Oikos, 209-219.
9
H UDEWENZ , A., & A. M. K LEIN (2013): Competition between honey bees and wild bees and the role of
nesting resources in a nature reserve. Journal of Insect Conservation, 17(6), 1275-1283.
L AUTENBACH , S., SEPPELT , R., L IEBSCHER , J., & C. F. DORMANN (2012). Spatial and temporal trends of
global pollination benefit. PloS one, 7(4), e35954.
L IERE , H., J HA , S., & S. M. P HILPOTT (2017): Intersection between biodiversity conservation, agroecology, and ecosystem services. Agroecology and Sustainable Food Systems, 41(7), 723-760.
P OTTS , S. G., IMPERATRIZ-FONSECA , V., NGO, H. T., AIZEN , M. A., B IESMEIJER , J. C., B REEZE, T. D., V
D ICKS , L., GARIBALDI , L. A., H ILL, R., S ETTELE , J. & A. J. V ANBERGEN (2016): Safeguarding Pollinators
and their Values to Human Well-Being. Nature, 540(7632), 220-229.
REIF, A., & R. A CHTZIGER (2014): Shrubs, hedges, woodland coppices, field copses (shrub formations). Handbuch Naturschutz und Landschaftspflege, 1-45.
RUNDLÖF, M., N ILSSON, H., & H. G. SMITH (2007): Interacting effects of farming practice and landscape context on bumble bees. Biological conservation, 141(2), 417-426.
RUNDLÖF, M., ANDERSSON, G. K., BOMMARCO, R., FRIES, I., HEDERSTRÖM, V., HERBERTSSON ,
L., JONSSON, O., KLATT, B. K., PEDERSEN, T. R., YOURSTONE, J. & H. G. SMITH (2015): Seed
coating with a neonicotinoid insecticide negatively affects wild bees. Nature, 521(7550), 77- 80.
SHARAF, H. (2018) (Diss.): Vegetation studies in an agroforestry system with value wood production: analysis of nature conservation potentials of different seeding and treatment methods as well as the interaction between value wood, herbaceous vegetation and agricultural land. University of Freiburg.
STEFFAN-D EWENTER, I., & T. TSCHARNTKE (2000): Resource overlap and possible competition
between honey bees and wild bees in central Europe. Oecologia, 122(2), 288-296.
WESTRICH, P., FROMMER, U., MANDERY, K., RIEMANN, H., RUHNKE, H., SAURE, C. & J. VOITH
(2011): Red List and overall species list of bees (Hymenoptera, Apidae) of Germany. - In: Binot-Hafke, M., Balzer, S., Becker, N., Gruttke, H., Haupt, H., Hofbauer, N., Ludwig, G., Matzke-Hajek, G. & M. Strauch (Ed.): Rote Liste gefährdeter Tiere, Pflanzen und Pilze Deutschlands. Volume 3: Invertebrates (Part 1). - Münster (Landwirtschaftsverlag). - Naturschutz und Biologische Vielfalt. 70 (3), 373-416.
WESTRICH , P. (2018): The wild bees of Germany (Vol. 4100). Stuttgart.
WOJCIK, V. A., M ORANDIN , L. A., D AVIES A DAMS , L., & K. E. R OURKE (2018): Floral resource competition
between honey bees and wild bees: is there clear evidence and can we guide management and conservation?. Environmental entomology, 47(4), 822-833.
Y ACHI , S. & M. LOREAU (1999): Biodiversity and ecosystem productivity in a fluctuating environment: the
insurance hypothesis. Proceedings of the National Academy of Sciences, 96(4), 1463-1468.